Improve Fine Needle Aspiration (FNA) Biopsies by Eliminating a Common Step

Fine needle aspiration biopsies (FNAs) are common procedures performed by physicians around the world, in facilities large and small. An FNA procedure utilizes thinner needles (21-27 gauge) to extract tissue samples from a patient's discovered lesion, which pathology uses to diagnose whether the lesion is malignant or benign1. FNAs can be done on many anatomic sites, but are typically performed on a lymph node, thyroid gland, breast, liver, or pancreas 2.
Once the specimens are extracted by the physician, they are smeared on microscopic slides using a variety of techniques and are then sent to cytopathology for evaluation.
Clinicians will often utilize ultrasound to locate the lesion and guide the needle during an FNA procedure. When ultrasound is used for an FNA procedure, the ultrasound probe should be covered with a sterile viral barrier, and sterile ultrasound gel is likely used while scanning.
While the use of ultrasound gel is ubiquitous with ultrasound imaging, radiology and endocrinology physicians performing FNA biopsies and pathologists evaluating the samples should be aware of the complications gel can create in their ability to adequately diagnose the patient.
Let's look at a few studies that researched the effect of ultrasound gel on FNA biopsy artefacts:
- Effect of ultrasound transmission gel on ultrasound-guided fine needle aspiration cytological specimens
of thyroid, Cytopathology, 2012
Researchers tested 20 thyroid nodules (within 14 patients) by performing ultrasound-guided FNA biopsies. Four specimens per thyroid nodule were collected: two after removal of the gel used in the prebiopsy ultrasound and cleansing with chlorhexidine wash and two using sterile ultrasound gel.
"On slides collected with gel, cytological artefacts were detected in 60-65% of cases compared with 10-15% of cases without gel... Two of the 14 patients required repeated FNA due to non-diagnostic cytology results caused by inadequate sampling and gel-induced artefacts."
The degree of gel contamination in the artefacts ranged from a thin layer over the specimen to a thick layer that fully masked the specimen. The study included images of artefacts that "also mimicked and adhered to groups of colloid" (see Figure 1d).
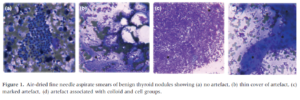
Cytopathy, 2012
The study concluded that ultrasound gel can impair the visibility of cells in the FNA specimen, making the diagnosis more difficult or resulting in a non-diagnosis3.
- Cell lysis due to ultrasound gel in fine needle aspirates; an important new artefact in cytology, Cytopathology,
1994
A breast fine needle aspiration sample contained artefacts due to contamination by ultrasound gel. Six additional specimens were collected from lymph nodes and breast, and half of the aspirates taken per case were contaminated with ultrasound gel.
"All cases with gel contamination showed artefactual changes," but the effect of those changes varied. Minor changes to the artefacts showed swelling of the cell nucleus and cytoplasm. Some contaminated artefacts resulted in "more dramatic cell swelling and leakage of nuclear chromatin into the cytoplasm." The most severe of artefacts resulted in exploded nuclei and cell lysis.
The study identified two key risks of ultrasound gel creating artefacts in FNA specimens:
Severe contamination can cause cell lysis, resulting in a non-diagnostic sample. Not only is a repeat procedure inconvenient for the clinician, it is not ideal for the patient to have to endure another intervention.
Even with cases of slight contamination, the pathologist may not know the specimen contains artefacts and may misinterpret the sample, resulting in a false positive diagnosis4.
- Ultrasound gel causes fine needle aspiration artifact? A clear choice, Acta Cytologica,
2012
FNA biopsy samples were collected from the thyroid and parotid glands of a fresh human cadaver, using ultrasound gel. Eighteen samples were collected and analyzed by cytopathologists.
The research showed "significant artifacts mimicking apoptosis, necrosis or colloid, making it difficult to visualize the cellular components and differentiate the artifact from the thyroid colloid." The findings point out "the potential to yield uninterpretable specimens or misreading if the artifact is not appropriately recognized 5."
Ultrasound-guided FNA biopsies have many advantages - they are minimally invasive and can provide a fast and accurate diagnosis. However, if the specimens are contaminated with ultrasound gel and result in misdiagnosis or a non-diagnostic sample, both clinicians and patients are inconvenienced.
One solution to improving ultrasound-guided FNA procedures is to remove the use of gel, and it's now possible with Envision™ ultrasound probe covers from CIVCO.
 Envision probe covers and scanning pads enable 100% gel-free ultrasound procedures, activated with a sterile liquid such as saline. Removing gel from ultrasound-guided FNA biopsies with Envision can help improve the visibility of cells in FNA specimens.
Envision probe covers and scanning pads enable 100% gel-free ultrasound procedures, activated with a sterile liquid such as saline. Removing gel from ultrasound-guided FNA biopsies with Envision can help improve the visibility of cells in FNA specimens.
 Envision covers can be used during other ultrasound-guided needle interventions, including CVCs and PICCs, to reduce the risks of potential harmful contamination posed by ultrasound gel (ultrasound gel consists of a combination of water and propylene glycol, a substance that has documented susceptibility to contamination). A unique configuration, the Envision pad, adheres to the patient or the ultrasound probe to scan non-intact or sensitive areas, including on neonates or post-surgical wound sites.
Envision covers can be used during other ultrasound-guided needle interventions, including CVCs and PICCs, to reduce the risks of potential harmful contamination posed by ultrasound gel (ultrasound gel consists of a combination of water and propylene glycol, a substance that has documented susceptibility to contamination). A unique configuration, the Envision pad, adheres to the patient or the ultrasound probe to scan non-intact or sensitive areas, including on neonates or post-surgical wound sites.
While ultrasound gel is itself made of a combination of water and propylene glycol, Envision viral barriers are designed with an adhesive on one side and a hydrophilic coating on the other, which allows for 100% gel-free ultrasound scanning.
The silicone adhesive can be safely adhered to, and eventually removed from, the patient or the probe due to its relatively low peel force. A sterile liquid is used to activate the hydrophilic coating, which acts as an acoustic couplant and lubricant to allow for dynamic scanning.
Envision covers are made of our trusted CIV-Flex™ material and are available in a variety of lengths.
In addition to improving the quality of FNA specimens, the removal of gel from ultrasound procedures provides another key benefit: improved clinician workflow.
 The silicone adhesive easily and quickly attaches to the ultrasound probe face or patient
The silicone adhesive easily and quickly attaches to the ultrasound probe face or patient
- Simply reposition the adhesive over the probe face to remove air bubbles, if needed
- Following the procedure, remove the cover or pad from the probe or the pad from the patient by peeling off the adhesive - it's quick, safe, and won't leave residue on the transducer
- No gel to clean up before sending the probe for high-level disinfection
Visit CIVCO.com/envision to see how Envision works and to request a complimentary sample.
Just add water with Envision to reduce the risk of gel contamination in your facility and simplify clinician workflow.
References
- Gupta PK. Progression from on-site to point-of-care fine needle aspiration service: Opportunities and challenges. 2010;7:6. Published 2010 May 12. doi:10.4103/1742-6413.63195
- Weydert, J. and Cohen, M. (2003). Fine Needle Aspiration: Current Practice and Recent Developments. Laboratory Medicine, 34(12), pp.851-854.
- Lalzad, D. Ristitsch, W. Downey, A. F. Little and M. E. Schneider-Kolsky "Effect of ultrasound transmission gel on ultrasound-guided fine needle aspiration cytological specimens of thyroid" Cytopathology 2012, 23, 330-333
- J. Molyneux, S. B. Coghill "Cell Lysis Due to Ultrasound Gel In Fine Needle Aspirates; an Important New Artefact In Cytology" Cytopathology 1994 5, 41-45
- Royer MC, Davidson DD, Dimitrov RK, Kuo CY, Kokaska MS "Ultrasound gel causes fine needle aspiration artifact? A clear choice." Acta Cytol. 2012;56(2):146-54





