A Breakthrough in New Ultrasound Probe Cover Research

 The required use of a probe cover for every endocavity ultrasound procedure has been well established by standards set by governing bodies and industry associations. And the use of an intracavity probe cover does not change the necessity for high-level disinfect or store the endocavity probes properly post-procedure.1,2 When considering which endocavity cover to use, questions that often arise include:
The required use of a probe cover for every endocavity ultrasound procedure has been well established by standards set by governing bodies and industry associations. And the use of an intracavity probe cover does not change the necessity for high-level disinfect or store the endocavity probes properly post-procedure.1,2 When considering which endocavity cover to use, questions that often arise include:
- Are probe covers reliable?
- What can or should be used as a probe cover?
- Are condoms better than probe covers?
Are Probe Covers Reliable?
Interestingly enough, this question has not been well researched - until now. Since 1995, there have only been two published studies that attempted to answer this question:
 "High Rates of Perforation are Found in Endovaginal Probe Covers Before and After Oocyte Retrieval for In-Vitro Fertilization Embryo Transfer," Published in 1995 by M. Hignett, et al in The Journal of Assisted Reproduction and Genetics3
"High Rates of Perforation are Found in Endovaginal Probe Covers Before and After Oocyte Retrieval for In-Vitro Fertilization Embryo Transfer," Published in 1995 by M. Hignett, et al in The Journal of Assisted Reproduction and Genetics3  "Comparison of Probe Sheathes For Endovaginal Sonography," Published in 1996 by V Rooks, et al in The Journal of Obstetrics and Gynecology4
"Comparison of Probe Sheathes For Endovaginal Sonography," Published in 1996 by V Rooks, et al in The Journal of Obstetrics and Gynecology4
When you look closely at these two studies, which are often referenced in high-level disinfection guidelines, there are some serious challenges with methodologies, sample sizes, and other aspects. Most important to note is that the covers and condoms used in both studies are no longer manufactured. Additionally, the standards and quality assurance practices required for the manufacturing of probe covers have dramatically improved since 1995.
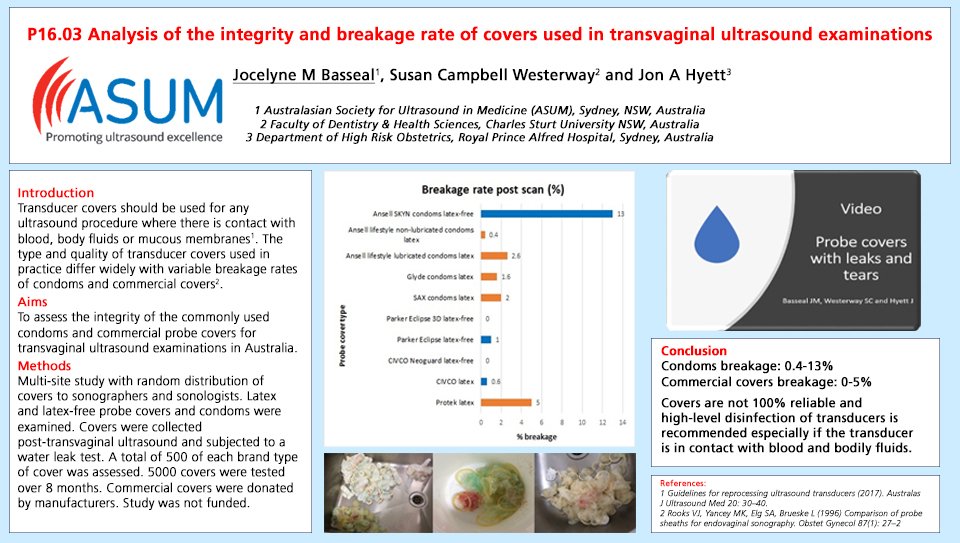
A more recent investigation into the reliability of commercially available probe covers was undertaken in 2018 by Dr. Jocelyne Basseal and her colleagues, Dr. Susan Westerway and Professor Jon Hyett, of Australia. They evaluated 5 different medical grade endocavity probe covers (500 of each) and 5 different condoms (500 of each) for a total of 5,000 covers and condoms tested.
The researchers discovered the commercial endocavity cover breakage rate ranged from 0.0 to 5.0%, and that the condom breakage rate ranged from 0.4 to 13.0%5. This is a significant finding, as it is a dramatic difference from the commercial endocavity cover breakage rate of 8.0-81.0% reported in the earlier studies referenced so often today in national and international guidelines.3,4
Further, this new endocavity probe cover study found commercial covers were more reliable than condoms, which is also a significant change from the earlier studies.
Specifically, the CIVCO NeoGuard® endocavity covers tested showed no breaks (0/500), and CIVCO latex endocavity covers reported a breakage rate of 0.6% (3/500).
The results of this study were first reported at the 2018 International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) Annual Scientific Meeting in Singapore as an electronic poster.5 In this original poster presentation, five different commercial covers, (500 of each) and five different condoms (500 of each) were tested for post-procedure breakage. Macroscopic and microscopic tears, which are not visibly seen when removed from the transducer, were evaluated with a water leak test. The results can be seen in the copy of the poster below.
These findings were later presented at the 2019 Asia Pacific Society of Infection Control (APSIC) Annual Meeting in Vietnam6 and were accepted for publication in 2019 in Infection, Disease, and Health9.
Some of the major strengths of this study are:
- Not funded by industry: While individual manufacturers donated products for the study, the investigators were not paid for their time in collecting data or preparing for presentation or publication.
- Sample size: a significant sample of 500 of each cohort were tested for a total of 5,000 covers and condoms
- Multiple sites, sonographers, and sonologists participated in the study
- Operators were blinded to the cover or condom they were using, thus eliminating any user bias
What can and should be used as an intracavity probe cover?
Commercially manufactured endocavity probe covers are specifically made for one purpose. They have been tested as an effective viral barrier,8 are FDA cleared, and have a very stringent quality assurance standard. Use of anything else, such as condoms, is considered off-label use.
Are condoms better than probe covers?
Condoms are not manufactured as an ultrasound probe cover, are not FDA cleared as a probe cover, have not been validated as a probe cover, and as the recent probe cover study results conclude, are actually less reliable than commercially manufactured endocavity probe covers.
Meet Dr. Jocelyne Basseal:

Dr. Basseal holds a PhD in Microbiology and has developed a passion for improving infection control practices in the ultrasound department on a global scale. She has published numerous peer-reviewed journal articles, and academic textbooks and is an invited international speaker and the principal author of the ACPIC/ASUM Guidelines for Reprocessing Ultrasound Transducers7. Dr. Basseal is involved in several ongoing research projects in resolving ultrasound infection prevention. She is the Managing Editor of The Australasian Journal of Ultrasound in Medicine (AJUM), as well as the Research Grants and Policy Manager for ASUM. She is an active STEM mentor and resides in Australia. You can follow her on Twitter @JocelyneBasseal
References
- "Guidance for Industry and FDA Staff - Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers." (September 10, 2015). www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm089001.htm
- W Rutala, David and the Healthcare Infection Control Practices Advisory Committee (HICPAC). "CDC Guideline for Disinfection and Sterilization in Healthcare Facilities" 2008.
- M. Hignett, P. Claman. "High Rates of Perforation are Found in Endovaginal Probe Covers Before and After Oocyte Retrieval for In-Vitro Fertilization-Embryo Transfer." Journal of Assisted Reproduction and Genetics, Vol 12, Number 9, 1995 Pages 606-609
- V. Rooks, M. Yancey, S. Elg, L. Brueske. "Comparison of Probe Sheathes For Endovaginal Sonography." Journal of Obstetrics and Gynecology, Vol 87, Number 1, 1996 Pages 27-29
- J. Basseal, S. Westerway, J.Hyett. Analysis of the integrity and breakage rate of covers used in transvaginal ultrasound examinations. Ultrasound in Obstetrics & Gynecology 2018; 52 (Suppl. 1): 138-193.
- http://apsic2019.com/upload/document/APSIC2019%20-%20Program%20V9%20Final%2013%20May%2015.3.pdf
- Basseal JM, Westerway SC, Juraja M, van de Mortel T, McAuley TE, Rippey J, et al. Guidelines for reprocessing ultrasound transducers. Australas J Ultrasound Med 2017; 20: 30-40
- http://viralzone.expasy.org/all_by_species.html
- Basseal JM et al., Analysis of the integrity of ultrasound probe covers used for transvaginal examinations, Infection, Disease & Health, https://doi.org/10.1016/j.idh.2019.11.003