The Joint Commission and Infection Control Compliance: Confusion or Confidence?


Patient safety and maintaining The Joint Commission (TJC) accreditation standards are two of the most important (if not THE most important) objectives of today's healthcare administrators and practitioners. However, they are often two of the most difficult objectives of which to achieve 100% satisfaction or compliance.
In fact, The Joint Commission released the top 5 requirements most frequently evaluated as "not compliant" through surveys and reviews completed in 2017 for hospital accreditation, and all present significant risks to patient and personnel safety:
| Non-compliance % | Standard/NPSG |
| 86% | LS.02.01.35 The hospital provides and maintains systems for extinguishing fires. |
| 73% | EC.02.05.01 The hospital manages risks associated with its utility systems. |
| 72% | LS.02.01.30 The hospital provides and maintains building features to protect individuals from the hazards of fire and smoke. |
| 72% | IC.02.02.01 The hospital reduces the risk of infections associated with medical equipment, devices, and supplies. |
| 70% | EC.02.06.01 The hospital establishes and maintains a safe, functional environment. |
Data collected through TJC National Patient Safety Goals (NPSGs), the Universal Protocol for Preventing Wrong Site, Wrong Procedure, Wrong Person Surgery™, and Accreditation and Certification Participation Requirements
One of the most common "non-compliant" standards, as identified by The Joint Commission, is the reduction of infections associated with medical equipment, devices, and supplies. 72% of hospitals seeking TJC accreditation in 2017 failed to meet this vital infection prevention and control standard. This published data is reflective of what we hear from our customers. Hospitals and clinics around the country see infection prevention as a major challenge for which they don't have a clear solution.
CIVCO Medical Solutions has been a leading designer and manufacturer of ultrasound accessories for over 35 years. We have worked closely with clinicians trying to solve infection control challenges to develop solutions that help them achieve and maintain compliance.
Our Confident Compliance promise takes a holistic approach to prevent infection in the usage of ultrasound.
1. Point of Care
 CIVCO offers a full range of ultrasound probe covers that serve as a viral barrier to protect against microbial migration, including viruses, bacteria, and bloodborne pathogens. Available for general purpose, vaginal and rectal, and transesophageal (TEE) probes, our transducer covers help reduce healthcare-associated infections.
CIVCO offers a full range of ultrasound probe covers that serve as a viral barrier to protect against microbial migration, including viruses, bacteria, and bloodborne pathogens. Available for general purpose, vaginal and rectal, and transesophageal (TEE) probes, our transducer covers help reduce healthcare-associated infections.
 Disposable transrectal and transvaginal needle guides are another point of care solution that can reduce risks associated with cross-contamination. Endocavity needle guides are designed to work with ultrasound systems' on-screen software guidelines, helping direct instruments for accurate placement in procedures such as tissue biopsies, fluid aspirations, catheterization, and marker placement.
Disposable transrectal and transvaginal needle guides are another point of care solution that can reduce risks associated with cross-contamination. Endocavity needle guides are designed to work with ultrasound systems' on-screen software guidelines, helping direct instruments for accurate placement in procedures such as tissue biopsies, fluid aspirations, catheterization, and marker placement.
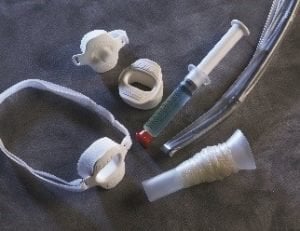 For transesophageal procedures, there are additional accessories to help protect patients and the TEE probe. CIVCO offers TEE bite blocks to protect against damage to the probe during an exam. Our TEE transducer holder attaches to most tables or surgical rails to hold the probe in a fixed location that allows for stable and accurate positioning during the procedure.
For transesophageal procedures, there are additional accessories to help protect patients and the TEE probe. CIVCO offers TEE bite blocks to protect against damage to the probe during an exam. Our TEE transducer holder attaches to most tables or surgical rails to hold the probe in a fixed location that allows for stable and accurate positioning during the procedure.
2. Transportation
 Daily transport of ultrasound probes can pose significant challenges to infection control and probe safety for any department since clean and dirty probes are transported throughout the facility multiple times a day. CIVCO's new TEE Probe Transport Tray helps you to be compliant with national transport standards and OEM guidelines.
Daily transport of ultrasound probes can pose significant challenges to infection control and probe safety for any department since clean and dirty probes are transported throughout the facility multiple times a day. CIVCO's new TEE Probe Transport Tray helps you to be compliant with national transport standards and OEM guidelines.
The Joint Commission's recommendation:
"Remove the device from the room in a covered container..."1
Probe manufacturers (OEMs) provide specific instructions as well: "To avoid damaging the shaft... Do not bend or coil the flexible shaft of the transducer in less than a 1-ft diameter circle"2
CIVCO's TEE Probe Transport Tray is disposable and made of recyclable water-resistant material. Its reversible clean/dirty lid simplifies your workflow for safe clean and dirty probe transport, and its special design reduces the risk of damage to the TEE probe and prevents cross-contamination.
3. Cleaning
 The Center for Disease Control (CDC) outlined probe cleaning and disinfecting guidelines as best practices to maintaining compliance. The CDC recommends pre-cleaning ultrasound probes classified as semi-critical prior to high-level disinfection:
The Center for Disease Control (CDC) outlined probe cleaning and disinfecting guidelines as best practices to maintaining compliance. The CDC recommends pre-cleaning ultrasound probes classified as semi-critical prior to high-level disinfection:
"Cleaning is the removal of visible soil... from objects and surfaces and normally is accomplished manually or mechanically using water with detergents or enzymatic products. Thorough cleaning is essential before high-level disinfection... because inorganic and organic material that remains on the surfaces of instruments interfere with the effectiveness of these processes."1
 The American Institute of Ultrasound in Medicine (AIUM) guidelines for pre-cleaning require a running water supply, dampened soft cloth with mild, non-abrasive liquid soap (i.e., an enzymatic cleaner), and a soft cloth to dry the probe2. CIVCO offers dry and enzymatic sponges, disinfecting wipes, and lint-free cloths to aid a compliant pre-clean process.
The American Institute of Ultrasound in Medicine (AIUM) guidelines for pre-cleaning require a running water supply, dampened soft cloth with mild, non-abrasive liquid soap (i.e., an enzymatic cleaner), and a soft cloth to dry the probe2. CIVCO offers dry and enzymatic sponges, disinfecting wipes, and lint-free cloths to aid a compliant pre-clean process.
We also recommend you reference your ultrasound manufacturers' IFU for probe-specific cleaning instructions.
4. High-Level Disinfection
The expectation of high-level disinfection for ultrasound probes is outlined by the CDC's recommendations:
"Probes such as rectal and vaginal, cryosurgical, transesophageal probes or devices also should be high level disinfected between patients."1
CIVCO has helped thousands of medical professionals learn these and other industry guidelines (including AIUMs) and best practices on ultrasound probe cleaning through our free webinar and blog series:
Automated Reprocessing
TEE Probe High-Level Disinfection
Top Tips for High-Level Disinfection of Ultrasound Probes: Part 1 and Part 2
 We can also help clinicians put these requirements and recommendations into practice with ASTRA, our automated high-level disinfection system for leading TEE and vaginal/rectal probes (including bi-plane probes up to 16 inches long). The ASTRA helps make compliance simple with:
We can also help clinicians put these requirements and recommendations into practice with ASTRA, our automated high-level disinfection system for leading TEE and vaginal/rectal probes (including bi-plane probes up to 16 inches long). The ASTRA helps make compliance simple with:
- Standardized Speed
- ASTRA can disinfect up to two probes at once
- Total reprocessing time is 10 to 15 minutes, depending on the type of probe, a disinfectant used and water pressure
- The system does not force into sleep mode during or after the reprocessing cycle, so staff do not need to monitor the process or be there to remove the probe immediately
- Significant Savings
- Reduced long-term costs by giving users the choice of four off-the-shelf, reusable high-level disinfectants: UltrOx™ (hydrogen peroxide), Revital-Ox™ RESERT® (hydrogen peroxide), Cidex® OPA, and MetriCide™ OPA
- No expensive equipment is needed for neutralization
- Helps protect expensive probes by not oversoaking in solution, not exposing them to high temperatures, and keeping probes stable and secure during the disinfection cycle
- Calculate how much you can save using the ASTRA TEE in your facility
- Confident Compliance
- Consumables and cycle data are automatically logged by the system to ensure compliance
- Clear, easy-to-follow steps guide users through the setup process
- For your facility to meet Intersocietal Accreditation Commission (IAC) requirements on leak testing your TEE probe, we provide an electrical leak tester and the ease of logging those pass/fail results directly within the ASTRA for each cycle
5. Storage
 The Joint Commission's recommendation of storing ultrasound probes and other semi-critical devices states:
The Joint Commission's recommendation of storing ultrasound probes and other semi-critical devices states:
"Store the device in a manner that will protect from damage or contamination...hanging vertically in a cabinet and storing in a clean environment."3
CIVCO offers storage cabinets for TEE, vaginal, rectal, and general purpose probes that fulfill the key storage requirements outlined by TJC to help you stay compliant:
- Shelves separate connectors from the probes
- Probes stored vertically
- Cords separated from the probes
- True HEPA filter with change indicator light when replacement needed
- Door locks for added security and a large viewing window gives a look into the cabinet without having to open the door
 TEE tip guards are also available to help protect your TEE probes during transport and storage in between procedures and cleaning cycles.
TEE tip guards are also available to help protect your TEE probes during transport and storage in between procedures and cleaning cycles.
Staying compliant with The Joint Commission is a major undertaking. Partner with CIVCO Medical Solutions to learn trends and guidelines, see potential solutions to your ultrasound probe infection control challenges and become Confidently Compliant.
6. Transportation
 Daily transport of ultrasound probes can pose significant challenges to infection control and probe safety for any department since clean and dirty probes are transported throughout the facility multiple times a day.
Daily transport of ultrasound probes can pose significant challenges to infection control and probe safety for any department since clean and dirty probes are transported throughout the facility multiple times a day.
CIVCO's new TEE Probe Transport Tray helps you to be compliant with national transport standards and OEM guidelines. Its reversible clean/dirty lid simplifies your workflow for safe clean and dirty probe transport, and its special design reduces the risk of damage to the TEE probe and prevents cross-contamination.
References
- CDC, "Guideline for Disinfection and Sterilization in Healthcare Facilities," 2008
- AIUM, "Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Probes Between Patients, Safe Handling, and Use of Ultrasound Coupling Gel," (Approved 5/16/2017)
- The Joint Commission, Infection Prevention and Control (IC) (Ambulatory Health Care/Ambulatory Health Care), 2017












